Hybrid Cochlear Implant Fills the Gap between Partial and Profound Hearing Loss
University of Cincinnati Medical Center one of only 15 test sites nationwide
Many clinicians used to think that cochlear implantation was only appropriate in patients with profound hearing loss, because it was believed that cochlear implants (CIs) were unable to preserve any hearing. In fact, until only recently (regardless of the implant manufacturer), there was only one type of electrode used in CI surgery, one in which the surgeon and audiologist did not attempt to utilize or preserve any residual hearing. However, a recent clinical trial has tested new CI technology, including the Nucleus® Hybrid™-L24 CI. Results of that study show that most patients implanted with the Hybrid-L24 retained their residual hearing.1 The success of this clinical trial allowed the FDA to grant approval for the Hybrid implant to be used in patients outside of the trial setting.
Director of the Cochlear and Auditory Brainstem Implantation Program, Ravi N. Samy, MD, elaborates on the changing needs of the hearing-impaired population: “The majority of my patients now, rather than being totally deaf, are those who have worn hearing aids for 10+ years, and now slowly their hearing is getting worse and worse, and they’re really struggling. They hear about cochlear implantation and get interested.”
He emphasizes, however, that it is important to perform accurate assessments on the onset and stability of hearing loss before implantation when evaluating candidates for electrode selection to potentially maximize the performance outcome for each patient1. The figure illustrates appropriate auditory frequency levels for Hybrid-L24 implant recipients.
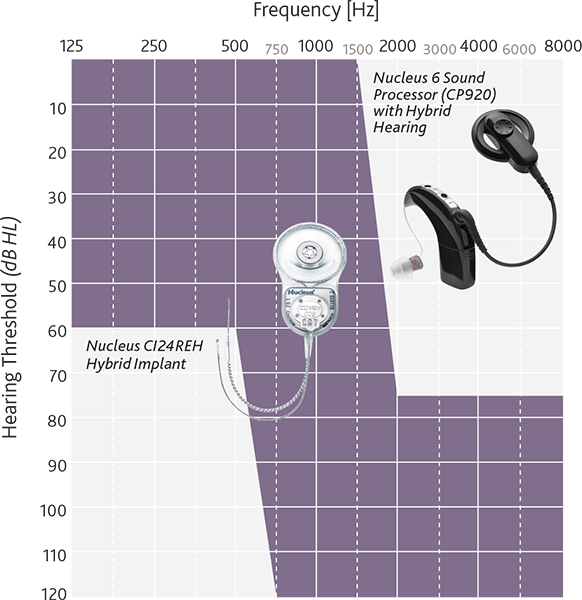
Picture provided courtesy of Cochlear Americas, © 2014 Cochlear Americas.
Participation in this trial has been far from the sole contribution of the University of Cincinnati Medical Center, however. In the last decade alone, the center’s research has benefitted from its partnerships with both Cincinnati Children’s Hospital Medical Center and the Cincinnati Veterans Affairs Hospital. As a result of this multidisciplinary focus, other physicians, audiologists, and surgeons look to the University of Cincinnati Medical Center for guidance with their most difficult cases.
Looking to the future, Samy says, “My goal in the next three to five years is to be one of the study sites that helps to bring a new technology to the United States – a totally implantable device. Several patients in Australia have already been implanted and evaluated. That would be truly revolutionary – not having anything external, having deaf people being able to hear 24/7.”
As for the long term, Samy foresees a time when CIs are as customized as contact lenses – using combined audiogram results, CT data, and MRI images of each patient’s ear to deliver 3-D prototypes of individually-designed CIs. Currently, the cost is prohibitive and the design challenges too difficult, but as Samy has seen first-hand, that will certainly change.
Reference:
1. Jurawitz M-C, Büchner A, Harpel T, Schüssler M, Majdani O, Lesinski-Schiedat A and Lenarz T. Hearing Preservation Outcomes with Different Cochlear Implant Electrodes: Nucleus® Hybrid™-L24 and Nucleus Freedom™ CI422. Audiol Neurotol 2014;19:293-309.
 Ravi N. Samy, MD, FACS
Ravi N. Samy, MD, FACS
Associate Professor of Otolaryngology
University of Cincinnati Cancer Institute, Brain Tumor Center, University of Cincinnati Neuroscience Institute, Neurosensory Disorders Center
Medical School: Duke University School of Medicine
513-475-8400

