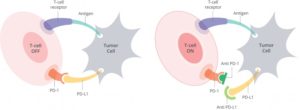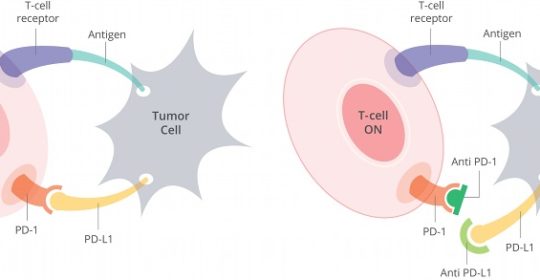
New Phase II Clinical Trial Tests Immunotherapy as Part of Standard Head and Neck Cancer Treatment
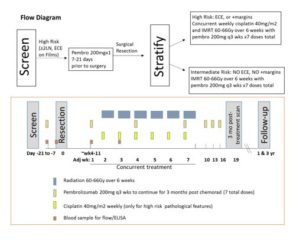
Schema for Phase II Trial of Adjuvant Cisplatin and Radiation with Pembrolizumab in Resected Head and Neck Squamous Cell Carcinoma. Image courtesy of Trisha Wise-Draper, MD, PhD.
Principal investigator Trisha Wise-Draper, MD, PhD, assistant professor of medicine, division of hematology/oncology, at the University of Cincinnati Medical Center, says she is “hoping for at least a 10% increase in the survival rate” for patients with head and neck squamous cell cancer in a Phase II trial of pembrolizumab being led by University of Cincinnati (UC) Medical Center. In earlier trials, these patients were treated with a combination of surgery, chemotherapy, and radiation but not immunotherapy.1-3 Jonathan Mark, MD, assistant professor of head and neck surgery, and a trial collaborator, is one of the surgeons who performs tumor resections, and terms pembrolizumab a very promising drug for locally advanced but resectable tumors.
UC Medical Center opened the Phase II trial, entitled: “Phase II Trial of Adjuvant Cisplatin and Radiation with Pembrolizumab in Resected Head and Neck Squamous Cell Carcinoma” in February 2016. (For information about inclusion and exclusion criteria, and primary and secondary outcomes, see https://clinicaltrials.gov/ct2/show/NCT02641093.)4 According to Dr. Wise-Draper, the first enrolled UC Medical Center patient is post-surgery and taking the study drug. She expects that a total of 80 patients will be enrolled at a total of six sites, with 15 to 20 patients at UC Medical Center.
Prior to the use of immunotherapy, treatment for head and neck squamous cell carcinoma was usually surgery and radiation or chemotherapy. In the Radiation Therapy Oncology Group 9501/Intergroup study, high-risk patients were randomized to radiotherapy alone or radiotherapy and cisplatin; disease-free survival was 58% at one year and 37% at three years.2 In the European Organization for Research and Treatment of Cancer Trial 229131, in which patients (with stage III or IV head and neck cancer) were randomized to radiotherapy alone or cisplatin and radiotherapy, investigators reported disease-free survival at one year of 72% and at three years of 40%.3
Dr. Wise-Draper’s trial involvement began with a letter of intent to Merck & Co., Inc., the manufacturer of pembrolizumab, in late 2014, suggesting that she conduct a trial of the drug in collaboration with Dr. Mark and other associates. According to Wise-Draper, the preclinical data that supports the trial proposal is based on work done by John C. Morris, MD, UC professor of medicine, and director, head and neck cancer and thoracic cancer program. Morris investigates the association of immunology and cancer, such as the effect of radiation or cisplatin on tumors and the resulting upregulation of PD-L1, the target of pembrolizumab.
As Dr. Wise-Draper explains, patients with head and neck squamous cell carcinoma who experience recurrent disease despite treatment with chemotherapy, radiation, and surgery must have resistance mechanisms. “Adding pembrolizumab to the treatment of these high-risk patients may overcome their resistance,” she says. Additionally, “we plan correlative studies that will look at T2 infiltration and T-cell function to try to understand resistance mechanisms to treatment and patients who will respond and those who will not,” Dr. Wise-Draper says. She anticipates Phase III trials if the current Phase II trial has promising results, and sponsored by a cooperative group, such as NRG Oncology, one of the lead protocol organizations in the National Clinical Trials Network.
Patients and physicians who are interested in UC Medical Center’s trial should call 513-584-7698.
References:
- Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27(10):843-850.
- Cooper JS, Pajak TF, Forastiere AA, et al, for the Radiation Therapy Oncology Group 9501/Intergroup. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1927-1944.
- Bernier J, Domenge C, Ozsahim M, et al, for the European Organization for Research and Treatment of Cancer Trial 22931. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945-1952.
- Phase II trial of adjuvant cisplatin and radiation with pembrolizumab in resected head and neck squamous cell carcinoma. Available at https://clinicaltrials.gov/ct2/show/NCT02641093. Accessed March 28, 2016.
 Trisha Wise-Draper MD, PhD
Trisha Wise-Draper MD, PhD
Assistant Professor of Medicine, Division of Hematology/Oncology
Head and Neck Oncology/Experimental Therapeutics
Associate Hematology/Oncology Fellowship Director
University of Cincinnati Cancer Institute
(513) 558-2826
wiseth@ucmail.uc.edu
 Jonathan Mark, MD
Jonathan Mark, MD
Assistant Professor of Head and Neck Surgery
(630) 650-3403
markjr@ucmail.uc.edu
Connect with Jonathan Mark, MD on Doximity
Leave a reply →